Although many studies have been dedicated to dendritic spines and their central synapses, or to neuromuscular junctions, we are aware of only two studies comparing specific morphological features of both these structures (Spacek, 1986; Couteaux and Spacek, 1988). Perhaps they were written too early to attract a greater attention.
Yet it seems that, at least in some cases, principles of ultrastructural architectonics are very similar in both locations not only in the main building scheme characterizing the chemical synapse (presynaptic axon terminal, synaptic vesicles, active zone, synaptic cleft, postsynaptic membrane with receptors, postsynaptic density) but also in some specific views as the shapes of dendritic spines and motor end plate interfolds and the dense plates in both of them.

Fig. 1: Examples of dendritic spine ultrastructure showing dense plates. A dendritic spine (left) may contain a spine apparatus with inner dense plates and outer dense plate radiating into the postsynaptic density (arrow). Other examples of outer dense plates (arrows) are also seen in the heads of other spines (middle,left). Scale = 0.2 µm. (From Spacek, 1985, with courtesy of Springer-Verlag ).
Specific similarities between the subsynaptic organization of dendritic spines and neuromuscular junctions:
Shape
In general, the 2D profiles of dendritic spines and motor end plate interfolds have the same building principle: narrower necks and wider tips. Their 3D shapes, however, are different: bulb-like in the dendritic spines and crest-like in the motor end plate (Fig. 2).
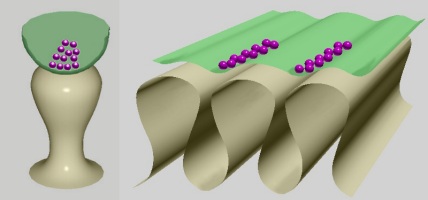
Fig. 2: Basic shape of dendritic spine (left) compared to that of the neuromuscular junction (right). The dendritic spine attaches to the dendrite with a narrow neck and receives a synapse on a bulb-like head. This lollipop shape is similar to the shape of the interfolds sectioned transversely. Opened axon terminal in green, synaptic vesicles in purple.
Dense Material
An electron-dense inner dense plate is formed between cisterns of spine apparatus derived from smooth endoplasmic reticulum in dendritic spines of larger (mushroom-shaped) type, radiating out as the outer dense plate and coming into contact with the postsynaptic density or with associated punctum adherens (Figs. 1 and 3).

Fig. 3: Comparison of dendritic spine (left) and motor end plate interfold (right) with their dense plate and strip (arrows). (From Couteaux and Spacek, 1988, with courtesy of Springer-Verlag.)
Smooth endoplasmic reticulum
Both these densities are more or less intimately associated with cisterns or tubules of smooth endoplasmic reticulum (differentiated into spine apparatus or sarcoplasmic triads).
Polyribosomes
Polyribosomes are present in subsynaptic region of both locations.
Vautrin and Mambrini (1989) suggested in accordance with a widely accepted hypothesis of that time, that narrower low parts of interfolds could change - like the necks in dendritic spines - the electric resistance and thus to regulate a propagation of the amplified postsynaptic signal into the muscle fiber. At the present time, apart from the voltage amplification function, dendritic spines are thought to represent molecular microcompartments influencing synaptic efficacy by controling the concentration of Ca2+ in the subsynaptic region, and to be a site of local proteosynthesis. We suppose that the motor end plate interfolds could have similar functional significances. The electron-dense material (which contains mainly actin in the dendritic spines but is of unknown composition in the interfolds) could take part in the maintenance of the shape of interfolds and its plastic changes. It possibly could even have a relationship to the maintenance of the postsynaptic density and membrane receptors, with the aid of polyribosome proteosynthesizing machinery, in the same way suggested in dendritic spines. The elements of smooth endoplasmic reticulum, which are known to be capable of sequestering Ca2+ in the dendritic spines, could have the similar function also in the motor end plate interfolds.
The similarities between spines and neuromuscular junctions offer intriguing possibilities for future studies. Elucidation of the above mentioned considerations are needed by ultrastructural analysis of unmasked cytoskeleton in motor end plates. Substructures so far detected in frogs only should be revealed also in mammalian postsynaptic interfolds. Microfluorometric detection of the concentration gradient of calcium during the generation of the action potential in the motor end plate should elucidate whether the microcompartment hypothesis is applicable not just to neuronal dendritic spines but also to the motor end plate interfolds.
References:
- Couteaux R (1981) Structure of the subsynaptic sarcoplasm in the interfold of the frog neuromuscular junction. J Neurocytol. 10, 947-962.
- Couteaux R, Spacek J: Specializations of subsynaptic cytoplasms. Comparison of axospinous synapses and neuromuscular junctions. In: H Zimmerman, ed., Cellular and Molecular Basis of Synaptic Transmission. NATO ASI Series, H21. Springer-Verlag, Berlin, Heidelberg, 1988, pp. 25–50.
- Pannese E: Neurocytology. Thieme Medical Publishers, New York, 1994.
- Peters A, Palay SL, Webster HD: The Fine Structure of the Nervous System: The Neurons and Supporting Cells. Philadelphia, PA: W.B. Saunders Co. (1991)
- Pospisilova B, Parizek J (1976) Comparative study of distribution of motor-end-plates in the muscles of the hind limb of some laboratory animals and the lower limb of man. Suppl. Sbor. ved. praci Hradec Kralove 19:411-422
- Spacek J (1985) Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat. Embryol. 171:235-243.
- Spacek J : Similarities between organization of subsynaptic cytoplasm at frog neuromuscular and mammalian axospinous junctions. Proceedings of the 2nd Czechoslovak – East German Bilateral Symposia of Anatomists, Histologists and Embryologists. Bratislava, 1986, p. 88.
- Vautrin J, Mambrini J (1989) Synaptic current between neuromuscular junction folds. J Theor Biol 140, 479-498.