Materials: Stoelting tissue slicer/chopper, new double edge razor blades, 70% ethanol, Kim-wipes, 4% to 7% melted Agar (45 to 50 degrees C), buffer, filter paper, artist's brush, plastic transfer pipettes, metal spatula, plastic Petri dishes, 0.1 M buffer (preferably phosphate buffer).
Procedure:
- The brain should have been previously rinsed 3 X in buffer solution, either in 0.1 M phosphate or 0.1 M cacodylate. Then, allow the brain to sit in same buffer until ready to use.
- Have ready freshly made 4% or 7% agar. See the protocol for agar preparation.
- Clean the base pad of the tissue chopper with 70% ethanol and wipe dry with Kim-wipe.
- Clean a new razor blade with 70% ethanol to remove any oils.
-
Prepare to attach the blade to the blade holder, located on the right side of the chopper arm. To do this, unscrew the two nuts located on the left side of chopper arm and remove them. This will release the blade holder. Note that the blade holder has two screws. See diagram:
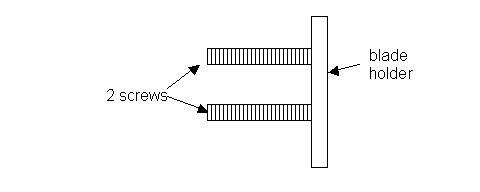
- Hang the razor blade onto the two screws via its central slit. Then insert the blade holder back onto the right side of the chopper arm. Secure the two nuts onto the two screws, but not tightly. Leave the blade holder a little loose.
- Lower the chopper arm onto the stage so that that blade edge rests on the stage. Now tighten both nuts to secure the blade holder. Raise the chopper arm and hold in place with a wooden stick until ready to use.
-
The stage moves to the left or to right, by turning the Vernier dial CCW or CW, respectively. The tissue, therefore, can be advanced for cutting, by moving the stage, either to the left or to the right. See diagram:
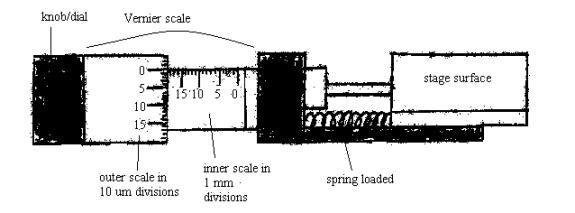
Examine the Vernier scale; the inner scale is divided into millimeters. Each division equals 1 mm. The outer scale/dial is divided into 10µm divisions, going from 0 to a total of 500 microns. Each revolution of the outer dial equals 500 microns. When turning this outer knob, CCW, the scale progresses from 0 to 500µm. This will move the stage to the left. See above diagram.
- Carefully transfer the tissue to a piece of filter paper, using the spatula or whatever is most appropriate. Add a few drops of buffer to keep the tissue wet and at the same time, moisten the area of filter paper.
-
Drop by drop, add the melted agar (45 to 50 degrees C) to the tissue via the plastic pipette, until a mound of agar is formed, completely covering the tissue. It might also be a good idea to create a support wall of agar around the tissue. Make sure there is no air bubbles in the agar. See diagram:

- Place the agar-covered tissue into a 4 degree C refrigerator for about 10 minutes to allow the agar to harden.
- When agar has hardened, place the tissue on the tissue chopper stage. Tape the filter paper down to the stage with paper tape. Moisten the filter paper with a little buffer in order to keep tissue moist.
- Move the stage, either to the left or to the right, to position the tissue for its first cut and subsequent slicing. Ideally, position the tissue so that the first cut starts at zero on the Vernier scale, but this is not crucial.
- Decide on section thickness, e.g., 300 um, and prepare to cut tissue into several or many 300 µm slices. Wet the razor blade a little with buffer before slicing. This may prevent the tissue from sticking to the blade during slicing.
- To slice tissue, bring slicer or chopper arm down into tissue quickly but then retract the arm slowly to remove. Make sure you slice right down to the filter paper layer. If tissue slice sticks to blade, remove tissue with wet brush (wet with buffer).
- Turn the Vernier CCW 300 µm (each small division equals 10 um) and then cut another slice, and so on.
- When the whole tissue has been sliced, then pick up each slice with the wet brush and transfer to appropriate container.
- When finished with tissue slicer/chopper, remove razor blade and dispose of it into a sharp's container.
- Clean the stage again with 70% ethanol. Store the tissue slicer/chopper away if appropriate.
- Dispose of extra tissue into biohazard bag or return it to a container of 0.1 M buffer for storage.