| |
Materials:
- Fine science knife
- #11 scalpel
- fine forceps
- two transfer pipets (broken Pasteur pipets)
- slides treated with rainexvibratome tools for fixed tissue
- with two broken slides glued to each end (depth of 2mm)
- with two coverslips glued to each end (depth of <400μm)
- two or three plain slides
- 24 well culture plate
- crazy glue
- amber glass jar
- 250mL glass beaker
- glass thermometer
|
| |
Making agar:
- Weigh out 0.7g low melting temp agar or agarose into glass jar and add 10ml water, shake.
- Place jar in beaker of water filled up to the shoulder of the jar and cap loosely
- Place beaker in microwave and place temperature probe in water
- Set temperature restriction on microwave to 80°.
- Microwave 2 min
- Agitate jar - agar should be melting and starting to boil
- Microwave another 2 minutes and shake again
- Microwave until agar is boiling and smooth - should only take 2-3 cycles. Add water to jar as necessary to keep agar fluid.
- Place beaker on hot plate set to keep water between 40-55° and place thermometer in beaker to monitor temp.
- Place stir bar in jar and keep agar well mixed throughout procedure.
|
| |
Vibratome prep:
- Use only tools designated for use on fixed tissue.
- Soak half of a double edged razor blade in acetone or alcohol to remove any oils or adhesives. Attach to blade holder.
- Pack vibratome chamber in ice and fill with phosphate buffer well in advance so that the buffer will be chilled.
|
| |
Slice dissection:
- Slide a small artist’s brush under the slice to gently remove it from the net, keeping it submerged. Discard net in aldehyde waste.
- Draw off fix with a pipette and replace with 0.1M cacodylate buffer.
- Rinse slice 5 times with cacodylate, waiting 5 minutes between each rinse.
- Replace cacodylate with 0.1M phosphate buffer and rinse 5 times.
|
| |
First agar embedding:
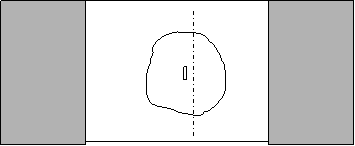
- Transfer slice onto slide with single slide spacers with transfer pipet. Leave slice in a small drop of buffer, enough to keep it well submerged.
- Using a small transfer pipet, remove all of the fluid from around the tissue.
- As quickly as possible, place a large drop of agar on the tissue with a transfer pipet and cover with a warm slide. Allow to cool at RT for a minute or so.
- Slide the small pipet between the slides and surround the agar with buffer and remove the top slide by gently pulling it sideways.
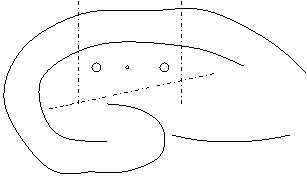
- Using a fine knife (Fine Science Tools), dissect out the area around the electrodes. Make one cut from alveus through CA1 just outside each stimulating electrode, then cut off dentate gyrus with one cut.
Second agar embedding:
- Place a plain slide on the hotplate with the label side off the plate to keep it warm.
- Remove and discard any excess tissue from the slide and turn the sample so that the electrode holes are down.
- Using a small transfer pipet, remove all of the fluid from around the tissue.
- As quickly as possible, place a large drop of agar on the tissue with a transfer pipet and cover with the warm slide. Allow to cool at RT for a minute or so.
- Slide the small pipet between the slides and surround the agar with buffer. Invert the slides and remove the top slide by gently pulling it sideways.
- Record which side of the section (left or right) is CA3.
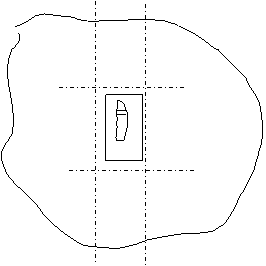
- Make two careful parallel cuts in the agar with the scalpel blade on either side of the tissue, as parallel to the sides of the section as possible. Cut away the top and bottom surrounding agar, leaving enough at the bottom to hold with forceps.
- Remove excess agar and grip bottom of sample with forceps. Turn the section 90˚ so that the tissue is suspended on its side. Place it on whichever side of the agar is cut most parallel with the tissue. Record which side is now up.
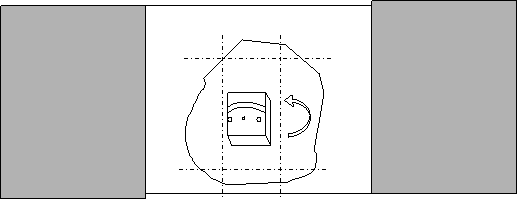
Third agar embedding:
- Warm another slide on the hotplate.
- Transfer the agar slice containing the tissue to the slide with double slide spacers. Keep track of which side is up.
- Place a drop of agar over the tissue and quickly cover with the warm slide. Allow to cool.
- Pipet buffer around the agar and remove the top slide.
- Using the scalpel, cut off the agar along one longitudinal side of the tissue, so the agar is in a half moon shape with the tissue at the flat edge.
|