Light Microscopy Photography: Nikon Labophot 2
4/18/02
Here is a procedure for taking light microscopic Kodak 35 mm color pictures either of tissue embedded in epoxy resin or of one-micron thick epoxy sections stained with 1% Toluidine Blue.
Materials:
Nikon Labophot 2 Light Microscope
Nikon N6000 35 mm camera
Cable release cord
200 ASA DX coded Kodak 35 mm color film
25 mm stage micrometer
Color temperature compensation filter (e.g., blue filter)
Air table
Nikon N6000 instruction manual
Nikon Labophot 2 instruction manual
Nikon’s “How to Use a Microscope and Take a Photograph” manual
Ross Lens Tissue
Can of Dust-Off
For the epoxy blocks photography, in addition to the blocks, have also a glass microscope slide (25 mm X 75 mm), round cover-glass (12 mm diameter) and forceps.
For Toluidine Blue-stained thick sections photography, you need the glass slide holding the stained sections.
Procedure:
1. The Kodak 200 ASA 35 mm color film. If it has been stored in the refrigerator, then allow it to come to room temperature, at least for 24 hours, before using.
2. The camera. Remove the camera from its attachment to the light microscope by pressing the lens release button (see p. 10 in manual for location of this button) and rotate camera clock-wise (CW) a quarter turn to lift it off the vertical tube adapter.
3. Setting automatic film speed for DX-coded film. (See p.22 in manual.)
-
-
Slide the power switch on the camera to ON position. See diagram below of the camera controls:
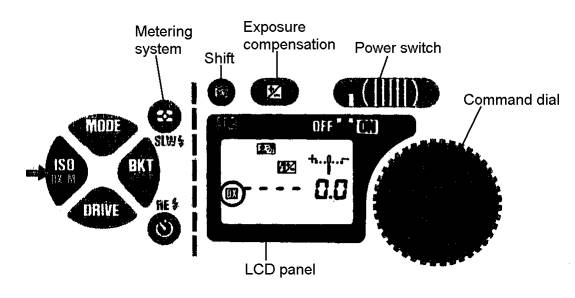
- While depressing the shift button, press film speed ISO button so that the letters DX are shown in LCD panel. The camera, from here on, will automatically detect film speed of DX-coded films. (To eliminate the DX auto setting, press ISO again, while depressing the shift button.) If film is already loaded into camera, you can simply verify the automatic speed setting by pressing the ISO button and see DX on the left side on the LCD panel.
-
4. Loading the camera with film. See pp. 13-15 in manual for instructions for film loading.
5. Checking/Setting shooting parameters. It is most likely that the previous user would have already installed the required parameters on this camera to shoot either epoxy resin blocks or the Toluidine Blue thick sections. To verify that this is the case, note the read-out on the LCD panel. See diagram below of LCD panel:
-
A.
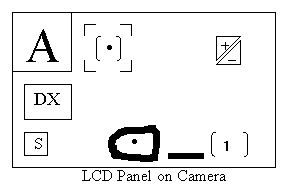
The above configuration indicates that the following parameters are already installed:a. DX coded
b. Single frame
c. Center weighted
d. Aperture Priority Auto
e. Bracketing mode on
f. Film loaded and set to 1st frame
B. If this is so, then proceed to instructions for shooting.
C. If, on the other hand these settings are not in place, then set these parameters for yourself as follows:
a. Automatic film speed for DX coded films. You should have already installed this, as described above in step C.
b. Set the film advance mode (p.25 in manual). To do this, depress and hold the Drive button. Then rotate the Command dial to S for single frame shooting, as seen on the LCD panel. See diagram above in step C.
c. Set the exposure metering system (see pp. 28-33 in manual) as follows:
i. Depress and hold the Metering System button (see above diagram in step C).
ii. Rotate the Command dial until you see the symbol Center-Weighted metering near the upper left corner of the LCD panel. See this Center-Weighted symbol below:
d. Set the Exposure Mode (see pp. 34-36 in manual). To do this:
i. Depress and hold the Mode button (see diagram above in step C).
ii. Rotate the Command dial until you see the letter A in the upper left corner on the LCD panel. This refers to the Aperture Priority Auto.
e. The next step is carried out during photography but will be described here and can be referred to. Prior to photographing an image, you can manually bracket for different exposure times (see pp. 52-53). This is referred to as setting the exposure compensation system. To do this, depress and hold the Exposure Compensation button (see symbol below and also in diagram in step C) and at the same time, rotate the Command dial from –5 to +5. Once set, the exposure compensation remains fixed until re-set.
6. Other Adjustments
-
- Place the light microscope atop the air table, if available. Inflate the air table. Using the air table reduces external vibrations during photography.
- Tape a small piece of black paper (1” X ½”) over the viewfinder eyepiece of the camera to block extraneous light (unless a piece is already there).
- Re-attach the camera to the vertical tube adapter.
- Set the Neutral Density #4 filter (ND 4) into place, excluding ND 2 and ND 16 filters.
- Set the microscope illumination lamp level just barely to the right edge of the little PHOTO rectangular label. See diagram:

7. Focusing the eyepieces at 10X magnification:
-
- Swing the 10X objective lens into position. Raise the condenser lens, if not already in position.
- Lower the stage a little and place one of your sample Toluidine Blue stained 1 um sections on the stage for viewing. If the slide is dusty or has fingerprints, then gently clean it with a Ross Lens tissue.
- Use the coarse, then fine, focusing knobs to bring the image into clear focus.
-
Rotate the right eyepiece diopter ring and observe the double crosshair configuration within the eyepiece. When in perfect focus for the right eye, the image should appear as distinct double lines. See diagram:

- In this same right eyepiece, bring the image of a stained section into sharp focus, using the fine focusing knob.
- To bring the left eyepiece into focus, rotate the diopter ring on the left eyepiece so until the image in the left eyepiece is in focus.
8. Kohler Alignment:
-
- Keep the 10X objective lens and condenser lens in place. Keep the same stained image in focus.
- Rotate the aperture diaphragm completely CW to open it.
- Rotate the base (field) diaphragm completely counterclockwise (CCW) to close it down to a small opening.
- Rotate the condenser-focusing knob (black knob under the stage over on the left side) until a crisp looking small octagon shape is obtained.
- Rotate the base (field) diaphragm CW slowly to open it and note if the octagonal image is off-center. To center the octagon, rotate one or both of the condenser centering screws (located under the stage, towards the front).
- Continue to enlarge the octagon image (rotating base diaphragm CW) until 90% of the field is in view. Continue to center this image. This image is referred to as the field diaphragm image.
- Finally, following the precise centering of the enlarged octagon, continue to open up the base diaphragm until the circumference of illumination is just a tad outside the viewing area. This completes the Kohler alignment. See diagram:

9. Other Adjustments:
-
- Remove the ND multi-filter holder and insert the blue color temperature compensation filter atop the field lens (only handle the edges of this blue filter to avoid fingerprints!!!). Next, replace the ND multi-filter.
- Attach the cable release cord to camera release terminal (see p. 4 in manual).
10. Photographing the 25 mm stage micrometer. In light microscopy photography, it is necessary to also photograph a magnification scale along with your samples so you will have a record of the magnifications used. Here we use a 25 mm stage micrometer containing millimeter (mm) and micrometer (um) divisions. You must photograph this scale at each magnification you use. It might be convenient to do this at the beginning of a photo session, so that you won’t forget about it later! See diagram below of this micrometer:
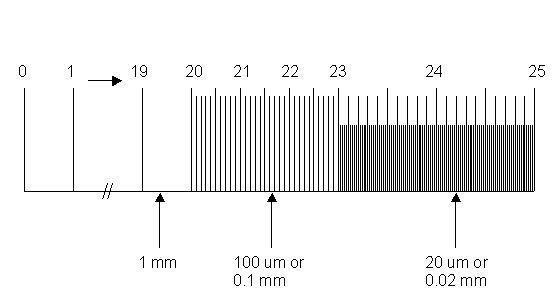
-
- Place the micrometer on the stage for viewing the numbers, from 0 to 25 mm, face-up and readable. Be aware that the divisions of this scale, either major or minor, will equal the same, regardless of what magnification the photograph is taken at. For example, the photographic image of the 20 um division (0.02 mm) will always equal 20 um, regardless of the magnification. Know that a particular unit of measure includes a white space plus one adjacent black bar.
-
For photography of the micrometer, you will maintain the follow parameters:
a. The Neutral Density filter #4 is always used.
b. For magnification photos at 10X, 20X, 40X and higher, always include the 20 micron (0.02 mm) scale end. For 2X magnification, include the 1 mm and 0.1 mm scale.
c. The Exposure Compensation (bracketing) setting is maintained at 0 bracketing (no bracketing).
-
To begin, let's start with 2X magnification. Therefore, move the condenser lens out of the way.
a. Have the aperture diaphragm wide open (completely CW).
b. Focus on the black lines of the micrometer scale, using the coarse, then fine, focus knobs as needed.
c. Close the aperture diaphragm somewhat (slightly CCW), until the blue peripheral image just disappears. This adjustment provides contrast and depth of focus.
d. Swing the binoculars all the way over to the left side, to remove them from the photo path.
e. When ready to shoot, press the button on the cable release cord. Do not touch the camera or air table during photography, to avoid any vibrations that would affect focus quality.
f. After the photo is shot, you may have to press the collar on the photo release cord, to release the button if it gets stuck.
g.When finished shooting, swing binoculars back into original position.
h. Keep a very careful written record of your parameters and subject on a multi-columned note sheet. See a sample of this written record at the end of this chapter.
-
Shooting the micrometer at 10X:
a. Switch from the 2X objective lens to the 10X.
b. Insert the condenser lens back into place (condenser lens is always used for 20X, 40X and higher magnifications, etc.).
c. Rotate the aperture diaphragm CW to completely open.
d. Focus on the smallest scale division discernible (20 um???).
e. Close down the aperture diaphragm (slightly CCW) until the level of light first begins to dim. This is the correct contrast level.
f. Swing the binoculars over the left.
g. Press the button on the cable release cord to shoot photo.
h. Swing binoculars back into place.
i. If ever you wish to re-shoot a photo because you feel it might be not right or out of focus, remember to re-focus with a wide open aperture diaphragm to get the most light needed to see well. Then close down the aperture diaphragm slightly to dim the surrounding light. That’s about it for shooting at 10X! If you wish to photograph the micrometer scale at higher magnifications, then proceed to the next step.
11. Shooting tissue embedded in epoxy blocks. Photographing the tissue in epoxy blocks will require shooting the image at several different exposure levels because it is difficult to know the one best exposure level. This is because the thickness of the epoxy block and/or tissue varies. The best approach may be to shoot the same block at the same magnification but at different exposure times (bracketing). For example, shoot a block at 2X but at -5, -4, -3, -2, -1, 0, +1, +2, +3, +4, +5 exposure. Then, repeat at the next higher magnification, e.g., 10X, etc. Keep these photographs as a reference to judge future blocks.
-
-
Place a block, tissue side up on a glass microscope slide on the stage. If you wish to photograph it at 2X, proceed as follows:
a. Lower the condenser lens for any 2X photos.
b. Keep the ND #4 filter in place.
c. Set the desired Exposure Compensation (bracketing) level (see step 5 in part E).
d. Open up the aperture diaphragm (CW).
e. Focus on the tissue as well as possible.
f. Close down the aperture diaphragm (CCW) until the blue peripheral image disappears.
g. wing binoculars to left.
h. Press button on cable release cord to shoot photo.
i. Repeat procedure at different bracketing levels. Just change the bracketing level and then shoot. No need to re-focus, unless you think you could improve the focus! In that case, swing binoculars into original position. Open up aperture diaphragm. Before adjusting the fine focus, note that the fine focusing knob has a scale denoted by 100 divisions (0 to 100). Note where the reference tick mark lies and record this value. Then adjust the focus. Try under- and over-focusing the image for photography. Record these new focusing values. -
When completely finished with this block, you may opt to maintain the 2X magnification but change to another epoxy block, for convenience sake. See diagram below of an epoxy block with rat hippocampal tissue.
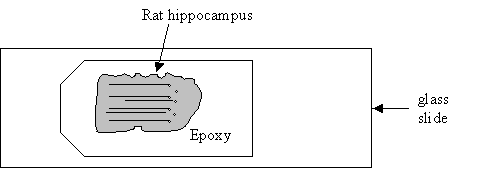
-
Shooting embedded hippocampal tissue at 10X.
a. Swing the 10X objective lens into place. Raise the condenser lens.
b. The aperture diaphragm should be wide open (completely CW).
c. Set the desired bracketing level (step 5 in part E).
d. Focus on the apical dendrites OR what I found to be helpful is focusing on the interneuron cell bodies in area Stratum Radiatum. You may have to take several photos at different focusing levels! See instructions for this in step i of part K 1.
e. Slightly close down (rotate CCW) the aperture diaphragm.
f. Swing binoculars over to the left.
g. Shoot the image.
h. Re-shoot at different bracketing levels/ focusing levels, if necessary. Otherwise, move on to the next magnification level. -
Shooting the embedded hippocampal tissue at 20X or 40X.
a. At 20X magnification (and also for 40X), you must place a small, round cover glass (12 mm diameter) upon the epoxy block! This should improve the resolution of the image. Use the fine forceps to handle this cover glass in order to avoid fingerprint oils.
b. The photography procedure of the embedded hippocampal tissue at 20X is now exactly the same as in the 10X magnification.
c. Shooting the embedded hippocampal tissue at 40X will follow the same procedure as shooting at 20X, except that all Neutral Density filters will be omitted. This will allow more light to be available at this high magnification.
-
12. Shooting Toluidine Blue-stained 1 um thick epoxy sections (of hippocampal tissue).
-
-
The photography procedure of 1 um stained sections is generally the same as in shooting the stage micrometer in that:
a. Neutral density filter #4 is always used at all magnifications.
b. The stage micrometer must also be photographed to correspond to the same magnifications being used in 1 um stained epoxy sections photography. -
What is different from the procedure used for the stage micrometer photography is:
a. The bracketing option should be used (Exposure Compensation system) to obtain the best pictures.
b. It is suggested that different focusing levels be tried.
c. It is suggested that the best feature to focus on at 10X, 20X and 40X, in hippocampal tissue, is the dendritic cytoplasm in the pyramidal apical dendrites in Stratum Radiatum. I think that the mitochondria in the dendritic cytoplasm is a good bet.
d. The camera itself may have to be rotated on occasion in order to match the orientation of the hippocampal tissue.
i. To do this, move the paper patch aside on the viewfinder of the camera.
ii. Swing the binoculars to the left.
iii. Look through the viewfinder and see that the rectangular photo field of the camera may not include all the hippocampal tissue, because the tissue happens to be oriented differently. See diagram below: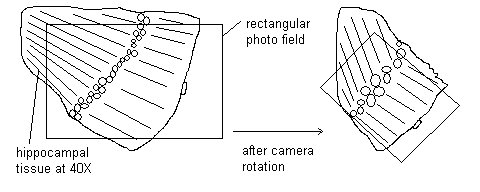
iv. To compensate, press the lens release button and rotate the camera until there is good alignment of the rectangular field and the tissue field. Replace the paper patch and continue with the photography procedure.
e. Follow the routine photography procedure as described in step J (for the 25 mm stage micrometer) in addition to the specific instructions described in step L.
-
13. Sample record sheet for light microscopy photography. This record-keeping of all photos taken was recommended by MicroVideo Instruments.
Subject: Tol Blue sections Date _____________
Kodak 35 mm color film, 200 ASA
Blue filter, Light P, Film A, meter setting – CW
| Photo #/ Subject | Mag | ND4 | Bracketing # | Fine Focus | Notes |
| Stage micrometer | 10X | ü | 0 | 10.5 | |
| Stage micrometer | 10X | ü | 0 | 9.0 | |
| Stage micrometer | 20X | ü | 0 | 20.2 | purple lines seen |
| Stage micrometer | 20X | ü | 0 | 22.0 | purple lines seen |
| Tol Blue slide #8 | 10X | ü | +3 | 92.0 | |
| Tol Blue slide #8 | 20X | ü | +4 | 70.0 |