by Josef Spacek
Most axons of peripheral nerves terminate on muscle cells. Whereas terminals of autonomic nerve fibers do not come in intimate contact with smooth muscle or gland cells, terminals of motor fibers form large synapses with muscle fibers, called neuromuscular junctions or motor end plates (Fig. 1).
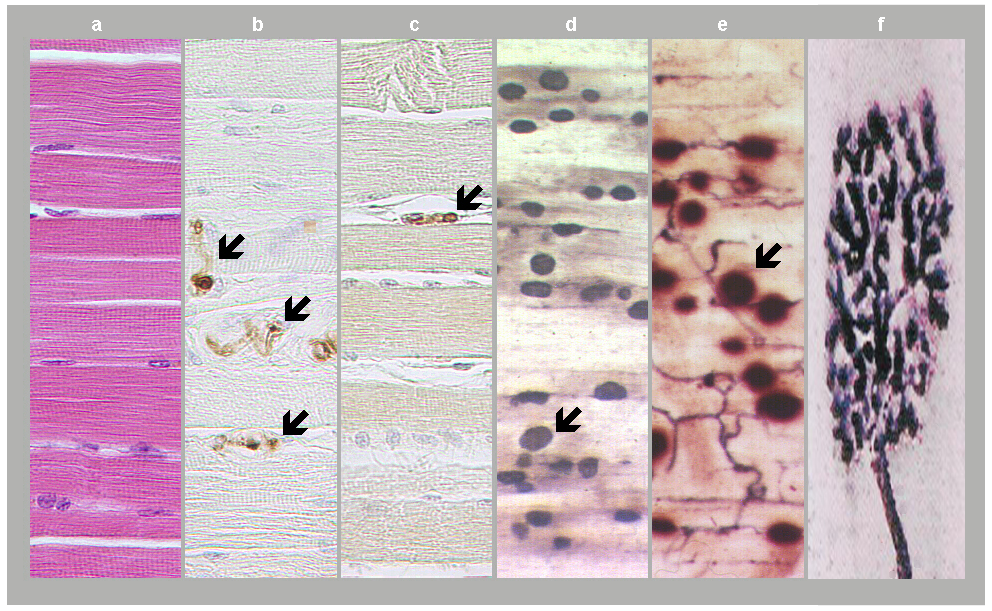
Fig. 1: Neuromuscular junctions. a) Skeletal muscle in standard HE staining. b) Motor axons in skeletal muscle as revealed in S100 protein immunostaining (arrows). c) Axon terminal as revealed in synaptophysin immunostaining (arrow). d) and e) Motor end plates (arrows) expressing acetylcholinesterase positivity and accumulated into innervation zones. f) Motor end plate in detail, adapted from David Ward, 2014. (Courtesy of Dr. Blanka Pospisilova, Dept. of Anatomy, Charles University Faculty of Medicine, Hradec Kralove).
These neuromuscular junctions are distributed in highly organized innervation zones in skeletal muscles (Fig. 2).
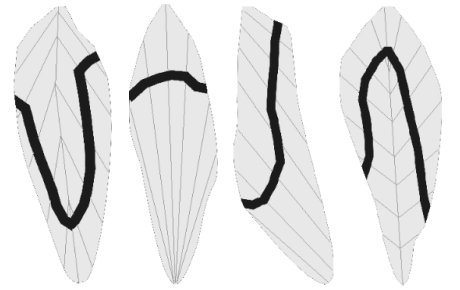 Fig. 2: Distribution zones of motor end plates (black lines) traced by positivity of acetylcholinesterase in human lower limb muscles (adapted from Pospisilova and Parizek, 1976).
Fig. 2: Distribution zones of motor end plates (black lines) traced by positivity of acetylcholinesterase in human lower limb muscles (adapted from Pospisilova and Parizek, 1976).
The moderately elevated motor end plate of skeletal muscle fiber forms a central depression with lamellar folds and grooves covered with the basal lamina (Fig. 3 and 4).
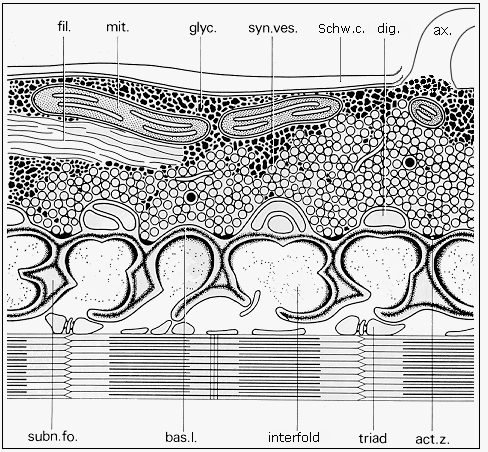 Fig. 3: Diagram of the ultrastructure of neuromuscular junction (adapted from Couteaux and Spacek, 1988, Fig. 8, with courtesy of Springer-Verlag): ax. - axon, fil. - neurofilaments, mit. - mitochondrion, glyc. -glycogen, syn. ves. - synaptic vesicles, Schw. c. - Schwann cell, dig. - terminal digitations of the Schwann cell, subn. fo. - subneural fold, bas. l. - basal lamina, act. z. - synaptic active zone
Fig. 3: Diagram of the ultrastructure of neuromuscular junction (adapted from Couteaux and Spacek, 1988, Fig. 8, with courtesy of Springer-Verlag): ax. - axon, fil. - neurofilaments, mit. - mitochondrion, glyc. -glycogen, syn. ves. - synaptic vesicles, Schw. c. - Schwann cell, dig. - terminal digitations of the Schwann cell, subn. fo. - subneural fold, bas. l. - basal lamina, act. z. - synaptic active zone
 Fig. 4: Electron micrograph of motor end plate. T - axon terminal, M - muscle fiber, arrow - foldings covered with basal lamina. Postsynaptic densities are apparent on tips of interfolds and missing in grooves. Scale = 0.3 µm. (Abdominal muscle, frog.)
Fig. 4: Electron micrograph of motor end plate. T - axon terminal, M - muscle fiber, arrow - foldings covered with basal lamina. Postsynaptic densities are apparent on tips of interfolds and missing in grooves. Scale = 0.3 µm. (Abdominal muscle, frog.)
In terminal branches of the motor axon covered with the Schwann cell, synaptic vesicles are accumulated along bands of active zones, an orientation of which corresponds with that of the folds (Fig. 5). The infoldings are numerous in mammals, very numerous in frog fast muscle fibres, capable of eliciting action potentials, sparse in frog slow muscle fibres, unable to generate action potentials and almost absent in fish muscles (Couteaux, 1981; Pannesse, 1994; Peters et al., 1991).
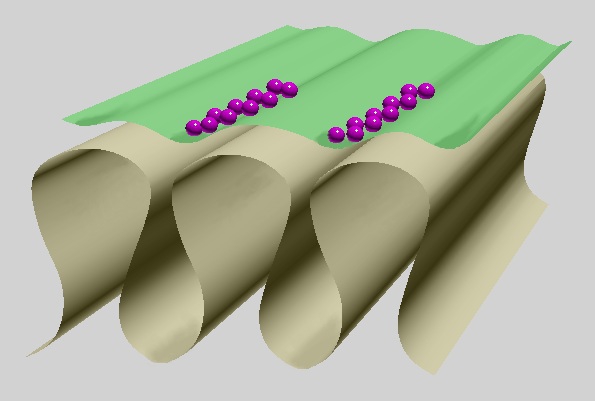 Fig. 5: Basic shape of motor end plate: infoldings with wider longitudinal crests and narrow lower interfold parts. Widely opened axon terminal in green, synaptic vesicles in purple, basal lamina omitted.
Fig. 5: Basic shape of motor end plate: infoldings with wider longitudinal crests and narrow lower interfold parts. Widely opened axon terminal in green, synaptic vesicles in purple, basal lamina omitted.
Two methodical approaches enhance cytoskeletal elements in motor end plates:
- After postfixation with 2% phosphate-buffered osmic acid, tissue blocks of both the cerebral cortex and skeletal muscle were treated with saturated aqueous solution of uranyl acetate on ice and ultrathin sections were double-stained with uranyl acetate and lead citrate.
- To unmask cytoskeleton, some tissue blocks of skeletal muscle were treated with 1% aqueous solution of Triton X-100 prior to postfixation and following procedure steps.
Using these methods an electron-dense axial strip of bottle-brush appearance is evident in the fast muscle fibres of frog, running in parallel with subsynaptic folds under the postsynaptic membrane (Figs. 6 and 7). Intermediate filaments surround the strips, thus forming subneural cylinders (Couteaux 1981).
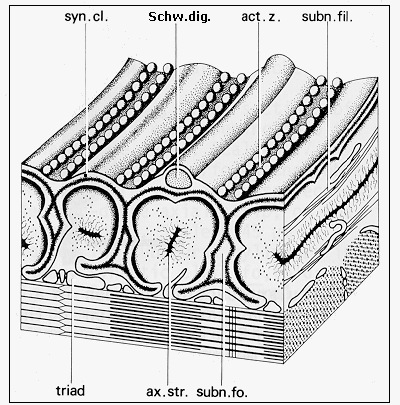 Fig. 6: Diagram of the ultrastructure of neuromuscular junction of frog (adapted from Couteaux and Spacek, 1988, Fig. 9, with courtesy of Springer-Verlag): syn. cl. - synaptic cleft, Schw. dig. - Schwann cell terminal digitation, act. z. - synaptic active zone, subn. fil. - subneural filaments, ax. str. - axial strip, subn. fo. - subneural fold
Fig. 6: Diagram of the ultrastructure of neuromuscular junction of frog (adapted from Couteaux and Spacek, 1988, Fig. 9, with courtesy of Springer-Verlag): syn. cl. - synaptic cleft, Schw. dig. - Schwann cell terminal digitation, act. z. - synaptic active zone, subn. fil. - subneural filaments, ax. str. - axial strip, subn. fo. - subneural fold
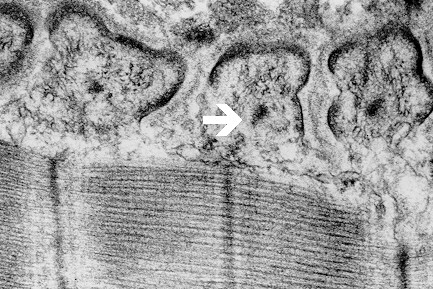 Fig. 7: Electron-dense axial strips (arrow) with surrounding intermediate filaments, unmasked by Triton X-100 in motor end plate interfolds of frog.
Fig. 7: Electron-dense axial strips (arrow) with surrounding intermediate filaments, unmasked by Triton X-100 in motor end plate interfolds of frog.
The ultrastructure of the neuromuscular junction exhibits several striking similarities to spine synapses in the brain.
- Couteaux R (1981) Structure of the subsynaptic sarcoplasm in the interfold of the frog neuromuscular junction. J Neurocytol. 10, 947-962.
- Couteaux R, Spacek J: Specializations of subsynaptic cytoplasms. Comparison of axospinous synapses and neuromuscular junctions. In: H Zimmerman, ed., Cellular and Molecular Basis of Synaptic Transmission. NATO ASI Series, H21. Springer-Verlag, Berlin, Heidelberg, 1988, pp. 25–50.
- Pannese E: Neurocytology. Thieme Medical Publishers, New York, 1994.
- Peters A, Palay SL, Webster HD: The Fine Structure of the Nervous System: The Neurons and Supporting Cells. Philadelphia, PA: W.B. Saunders Co. (1991)
- Pospisilova B, Parizek J (1976) Comparative study of distribution of motor-end-plates in the muscles of the hind limb of some laboratory animals and the lower limb of man. Suppl. Sbor. ved. praci Hradec Kralove 19:411-422
- Spacek J (1985) Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat. Embryol. 171:235-243.
- Spacek J : Similarities between organization of subsynaptic cytoplasm at frog neuromuscular and mammalian axospinous junctions. Proceedings of the 2nd Czechoslovak – East German Bilateral Symposia of Anatomists, Histologists and Embryologists. Bratislava, 1986, p. 88.
- Vautrin J, Mambrini J (1989) Synaptic current between neuromuscular junction folds. J Theor Biol 140, 479-498.