by Josef Spacek
The spine apparatus (SA) is a derivate of the smooth endoplasmic reticulum (SER). It is practically always present in dendritic spines possesing a perforated synapse, i.e. the mushroom spine or its sessile variety. A most typical location of the SA is the head of the spine but sometimes it can be found in a neck or -- in the case of the sessile spine -- in the dendritic shaft.
In its most characteristic constellation, one narrow tubule of the SER turns from the dendritic shaft into the neck of the spine to reach the head and branch into two or more stacked flat cisternae (Fig. 1). The larger synapse, the more cisternae. Among them, a dense material is present, called inner dense plates (Figs. 1, 2, 4). A fine filamentous material radiates from these plates to a vesicle-free punctum adherens-like nascent zone of the postsynaptic density (PSD) or occasionally, to the punctum adherens which binds an astrocyte process to the dendritic spine. We call this filamentous band an outer dense plate.
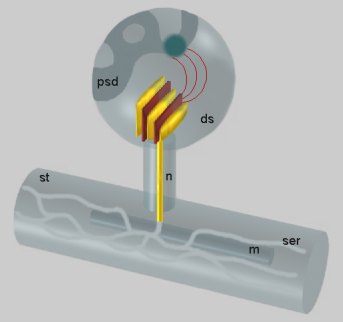 Fig. 1: Dendritic spine (ds) containing the SA. From the inner dense plates localized between the stacked cisterns (yellow) a filamentous material (red) radiates into the nascent zone (dark) of the postsynaptic density (psd). The narrow tubule running through the neck of the spine (n) connects the cisterns with the smooth endoplasmic reticulum (ser) of the dendritic shaft (st). An intimate apposition of the reticulum to a mitochondrion (m) is visible in the dendritic shaft.
Fig. 1: Dendritic spine (ds) containing the SA. From the inner dense plates localized between the stacked cisterns (yellow) a filamentous material (red) radiates into the nascent zone (dark) of the postsynaptic density (psd). The narrow tubule running through the neck of the spine (n) connects the cisterns with the smooth endoplasmic reticulum (ser) of the dendritic shaft (st). An intimate apposition of the reticulum to a mitochondrion (m) is visible in the dendritic shaft.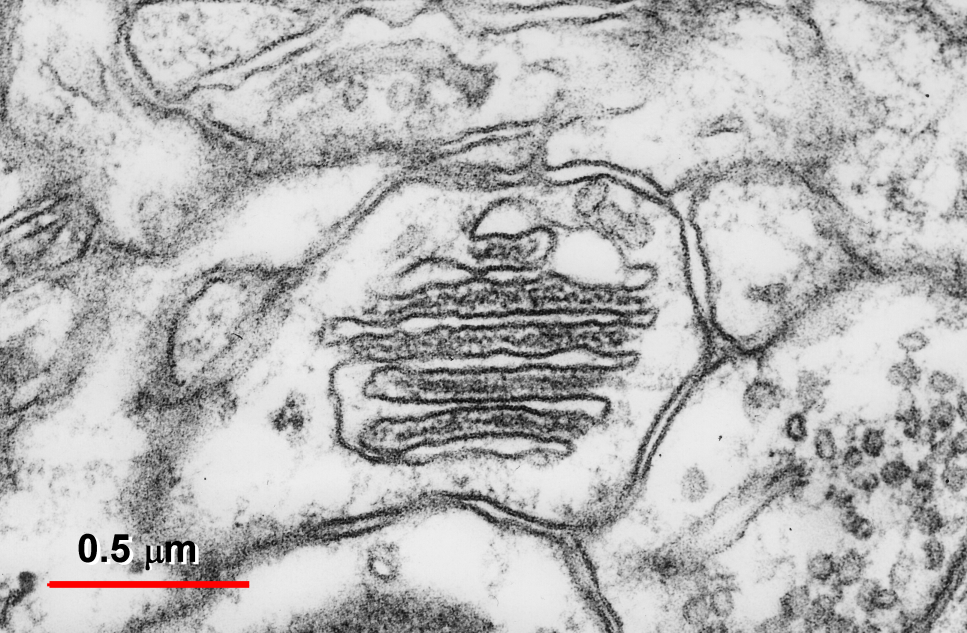 Fig. 2: Transversally sectioned SA formed by six flat cisternae and five inner dense plates.
Fig. 2: Transversally sectioned SA formed by six flat cisternae and five inner dense plates.
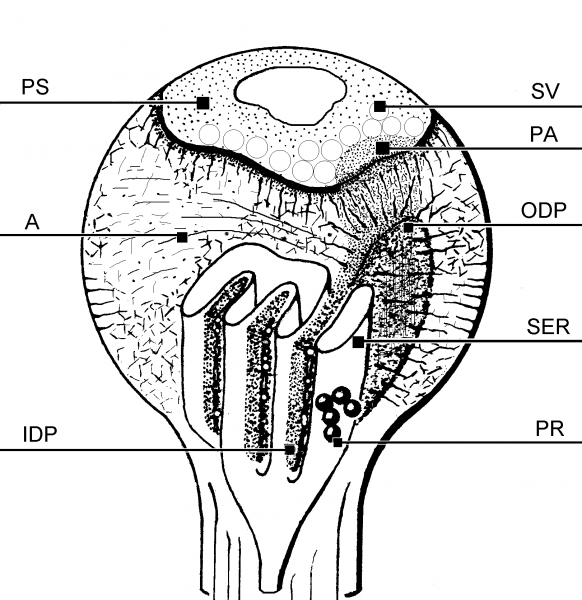 Fig. 3: A diagram of a dendritic spine possesing the SA. SER – cisternae of the endoplasmic reticulum, PR- polyribosomes, IDP, ODP – inner and outer dense plates, A – actin, PA – punctum adherens-like nascent zone, PS – postsynaptic density, SV – synaptic vesicles
Fig. 3: A diagram of a dendritic spine possesing the SA. SER – cisternae of the endoplasmic reticulum, PR- polyribosomes, IDP, ODP – inner and outer dense plates, A – actin, PA – punctum adherens-like nascent zone, PS – postsynaptic density, SV – synaptic vesicles
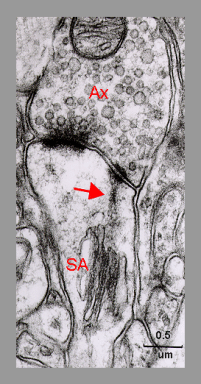 Fig. 4: An electron micrograph demonstrating the fine granulofilamentous material (arrow) radiating from the dense plates of the spine apparatus (SA) into the punctum adherens-like vesicle-free nascent zone of the postsynaptic density. Ax - axon terminal. ( Mouse neocortex. From Spacek, 1985b, with the courtesy of Springer-Verlag).
Fig. 4: An electron micrograph demonstrating the fine granulofilamentous material (arrow) radiating from the dense plates of the spine apparatus (SA) into the punctum adherens-like vesicle-free nascent zone of the postsynaptic density. Ax - axon terminal. ( Mouse neocortex. From Spacek, 1985b, with the courtesy of Springer-Verlag).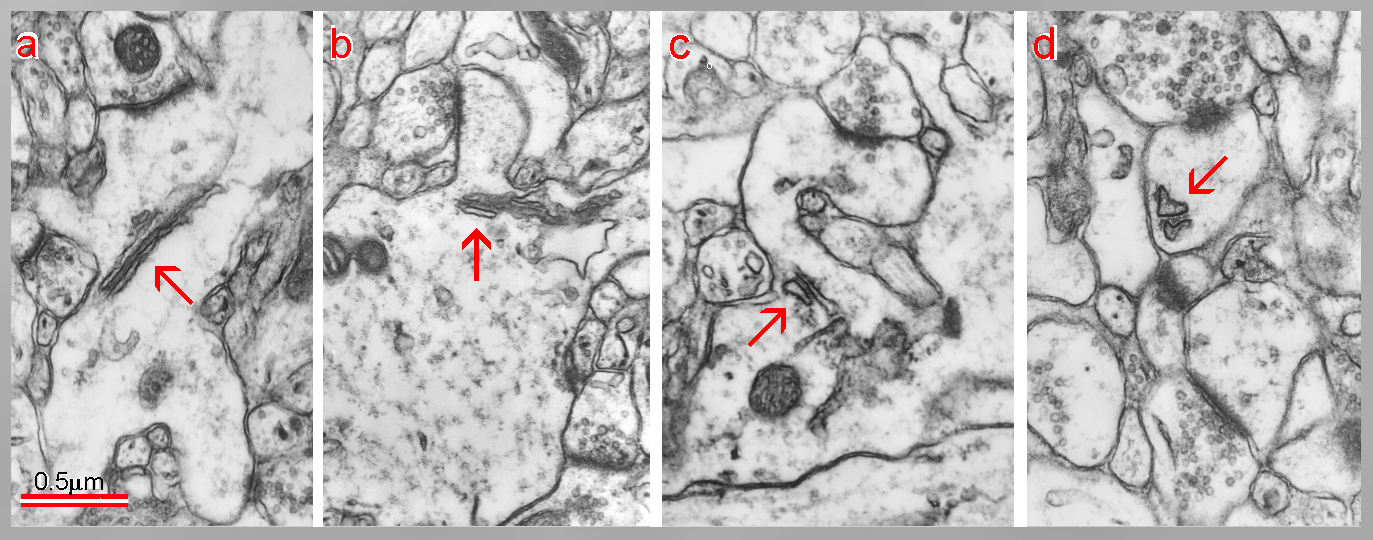 Fig. 5: Development of a dendritic spine from a sessile to a mushroom type. Confronting cisterns of endoplasmic reticulum enter from a dendritic shaft into a neck and head of the spine and form the SA (arrows). (Rat, hippocampus, postnatal day 15.)
Fig. 5: Development of a dendritic spine from a sessile to a mushroom type. Confronting cisterns of endoplasmic reticulum enter from a dendritic shaft into a neck and head of the spine and form the SA (arrows). (Rat, hippocampus, postnatal day 15.)
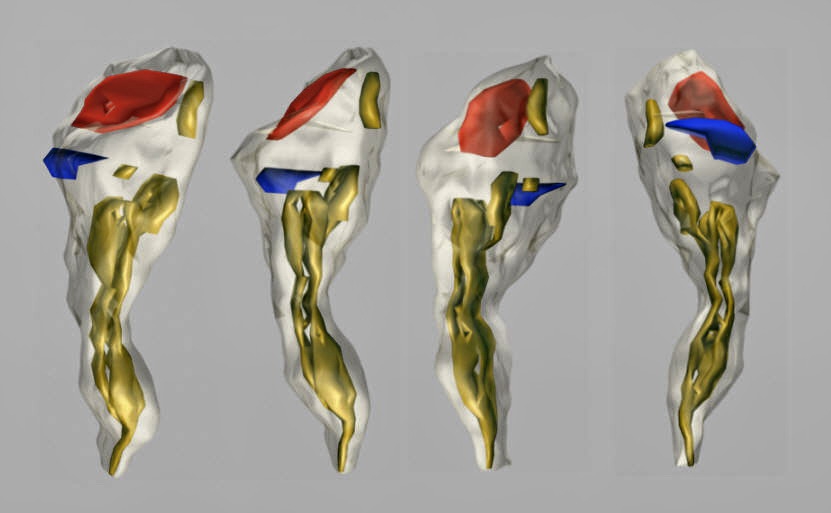
Fig. 6: A rotated three-dimensional reconstruction of the dendritic spine from the hippocampal stratum radiatum. A perforated excitatory synapse (red) and a macular inhibitory one (blue) are located on the head of the spine. The SA (yellow) is placed in the head and neck of the spine.
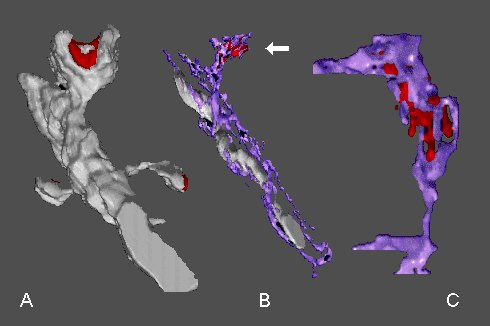 Fig. 7: A - a dendritic segment with mushroom-shaped spine possesing a perforated synapse (red) and with two thin spines. B - the mushroom-shaped spine contains the SA (arrow) with the inner dense plates (red). Note an intimate association between the smooth endoplasmic reticulum (purple) and mitochondrion. C - another SA with inner dense plates at higher magnification
Fig. 7: A - a dendritic segment with mushroom-shaped spine possesing a perforated synapse (red) and with two thin spines. B - the mushroom-shaped spine contains the SA (arrow) with the inner dense plates (red). Note an intimate association between the smooth endoplasmic reticulum (purple) and mitochondrion. C - another SA with inner dense plates at higher magnification
Actin, MAP2, proteinkinase, Ca2+, ATPase, calsequestrin, inositol 1,4,5 - triphospate 3 – kinase and synaptopodin are among substances which were referred in the literature to be present in the SA. We found in the inner dense plates ribosome-like particles, too. An exact significance of the SA is not clear. Its role in buffering (namely sequestring) Ca2+ in the dendritic spine compartment is supposed, but also a synthesis of a proteinaceous material constituting PSD (perhaps even receptors), especially during its extension in a process of the synaptic plasticity (e.g., LTP), is increasingly suggested.
Some authors incorrectly term as SA also profiles of smooth endoplasmic reticulum in spines of cerebellar Purkinje cell dendritic branchlets. Although an occasional parallel configuration of tubules or sacs of endoplasmic reticulum can remind the SA, any signs of higher differentiation and dense plates typical for the SA associated with perforated synapses are lacking in this location. Synapses on Purkinje cell dendritic spines are never of the perforated type.
An organelle identical in appearance with the SA (sometimes called a cisternal organelle in this location) is also found in the axon initial segment (Fig. 5). Its function there is completely unknown. Perhaps a comparative analysis of this organelle together in both these locations will help to uncover its functional significance. Another striking structural similarity exists between the outer dense plates and axial strips described in a subsynaptic cytoplasm of neuromuscular synapses (see Structure of Neuromuscular Junction chapter).
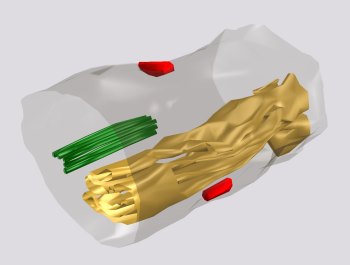 Fig. 8: A cisternal organelle located as an extraspinous variety of the SA (yellow) in an axon initial segment. Two axo-axonal synapses are placed on the axonal surface (red) and a bunch of linked microtubules, characterising the axon initial segment is present in its cytoplasm (green).
Fig. 8: A cisternal organelle located as an extraspinous variety of the SA (yellow) in an axon initial segment. Two axo-axonal synapses are placed on the axonal surface (red) and a bunch of linked microtubules, characterising the axon initial segment is present in its cytoplasm (green).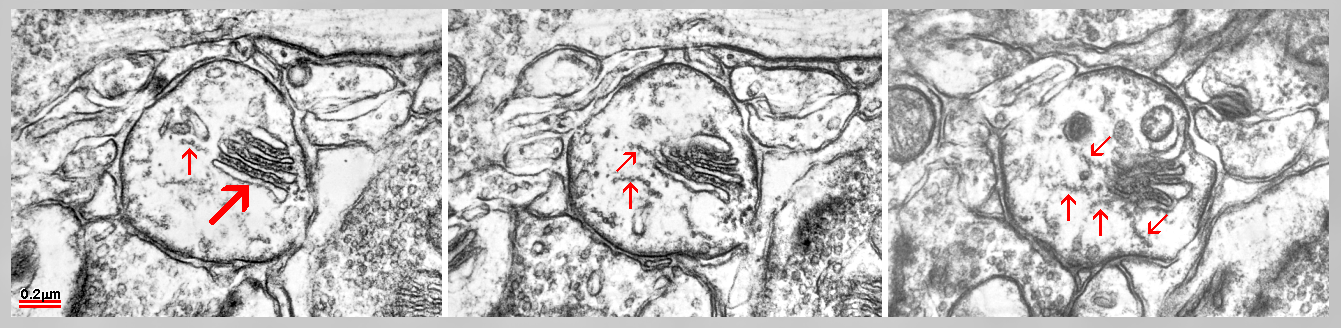 Fig. 9: Serial sections of a cisternal organelle located as an extraspinous variety of the SA in axon initial segment. Arrays of linked microtubules (arrows) look to radiate from inner dense plates. (Mouse cerebral cortex.)
Fig. 9: Serial sections of a cisternal organelle located as an extraspinous variety of the SA in axon initial segment. Arrays of linked microtubules (arrows) look to radiate from inner dense plates. (Mouse cerebral cortex.)
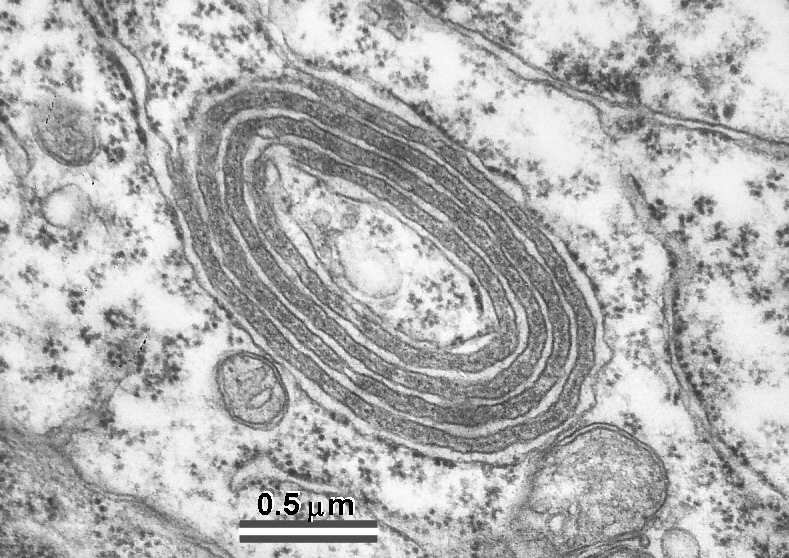 Fig. 10: Also peculiar organelles in thalamus show some structural similarities to the SA. They are located in a vicinity of so called filamentous contacts which are extensive puncta adherentia-like junctions characteristic of thalamic relay nuclei. (Rat, ventrobasal and lateral geniculate nuclei.)
Fig. 10: Also peculiar organelles in thalamus show some structural similarities to the SA. They are located in a vicinity of so called filamentous contacts which are extensive puncta adherentia-like junctions characteristic of thalamic relay nuclei. (Rat, ventrobasal and lateral geniculate nuclei.)
 Fig. 11: Occasional lamellar extensions (arrows) of the nucleus (N) called “nuclear pockets“ reveal a structural organization looking nearly identical with that of inner dense plates of the SA or with lamellar formations in thalamic neurons.
Fig. 11: Occasional lamellar extensions (arrows) of the nucleus (N) called “nuclear pockets“ reveal a structural organization looking nearly identical with that of inner dense plates of the SA or with lamellar formations in thalamic neurons.
Synaptopodin-deficient mice lack both the SA in dendritic spines and the cisternal organelle in the axon initial segment. It would be very informative to test an eventual relationship of synaptopodin to the thalamic and nuclear lamellar structures.
It is newly supposed that microtubules could contribute to the formation of the dense plates (or vice versa) and also that the SA has some biochemical features of the Golgi apparatus.
References
- Bell ME, Bourne JN, Chirillo MA, Mendenhall JM, Kuwajima M, Harris KM (2014) Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. J. Comp. Neurol. 522:3861-3864.
- Couteaux R, Spacek J. Specializations of subsynaptic cytoplasms. Comparison of axospinous synapses and neuromuscular junctions. In: Cellular and Molecular Basis of Synaptic Transmission. Ed.: Zimmerman H, NATO A50 Series, 25-50, 1988. Springer Verlag, Heidelberg
- Deller T, Merten T, Roth SU, Mundel P, Frotcher M (2000) Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J. Comp. Neurol. 418:164-181.
- Gray EG, Guillery RW (1963) A note on dendritic spine apparatus. J. Anat. Lond. 97:389-392.
- Karlsson U (1966) Three-dimensional studies of neurons in the lateral geniculate nucleus of the rat. I. Organelle organization in the perikaryon and its proximal branches. J. Ultrastr. Res. 16:429-481.
- Lieberman AR, Spacek J, Webster KE (1971) Unusual organelles in rat thalamic neurons. J. Anat. Lond. 109:365.
- Lieberman AR, Spacek J (1997) Filamentous contacts: the ultrastructure and thre-dimensional organization of specialized non-synaptic interneuronal appositions in thalamic relay nuclei. Cell Tissue Res. 288:43-57.
- Orth CB, Schultz Ch, Müller ChM, Frotcher M, Deller T (2007) Loss of the cisternal organelle in the axon initial segment of cortical neurons in synaptopodin-deficient mice. J. Comp. Neurol. 504:441-449.
- Pierce JP, Mayer T, McCarthy JB (2001) Evidence for a satellite secretory pathway in neuronal dendritic spines. Current Biology 11:351-355.
- Segal M, Vlachos A, Korkotian E (2010) The spine apparatus, synaptopodin, and dendritic plasticity. Neuroscientist 16:125:131.
- Shirao T, González-Billault Ch (2013) Actin filaments and microtubules in dendritic spines. J. Neurochem. 126:155-164.
- Spacek J (1985a) Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat. Embryol. 171:235-243.
- Spacek J (1985b) Relationships between synaptic junctions, puncta adhaerentia and the spine apparatus at neocortical axo-spinous synapses. Anat. Embryol. 173:129-135.
- Spacek J, Harris KM (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J.Neurosci. 17:190-203.
- Spacek J, Harris KM (1998) Three-dimensional organization of cell adhesion junctions at synapses and dendritic spines in area CA1 of the rat hippocampus. J.Comp.Neurol. 393:58-68.
- Westrum LE, Jones DH, Gray EG, Barron J (1980) Microtubules, dendritic spines and spine apparatus. Cell Tissue Res. 208:171-181.