by Rachel E. Ventura

The cell body of an astrocyte spans 10-20 microns and its processes radiate out for another 20-30 microns, forming the stellate glial cell. The stellate shape of the astrocyte can be appreciated in this light microscopic image.
Astrocytes fill between 4 and 8% of the volume of stratum radiatum in area CA1 of the hippocampus, making them the most predominant glial subtype there. In addition to astrocytes, the hippocampus is home to other glia, including microglia and oligodendrocytes.
An astrocytic presence near synapses seems to be important for synaptic function (see below). In this electron micrograph from rat hippocampus, astrocytes (blue) can be seen closely intermingling with synaptic complexes (arrows).
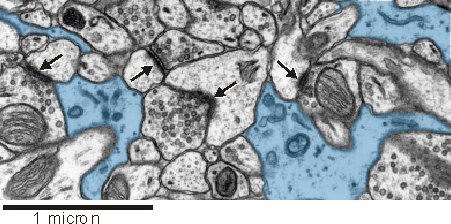
Astrocytes can be identified at the ultrastructural level by a number of key structural features:
- an irregular, stellate shape
- numerous glycogen granules
- bundles of intermediate filaments
- a relatively clear cytoplasm
Astrocytes, like other glial subtypes, have been commonly thought of as mere support and maintenance cells for the real actors in brain functioning, the neurons. Over the past several years, however, research has indicated a much more central role for astrocytes in non-synaptic as well as synaptic communication.
In synaptic transmission, astrocytes can exert an influence through modulation of the volume, composition, and the concentrations of ions, neurotransmitters, neuromodulators, and energy sources within the extracellular space. The involvement of astrocytes in message transmission motivates a reconsideration of the synaptic unit, as possibly including not only the pre- and post- synaptic partners but also neighboring glia.
Astrocytic syncytium: a means of non-synaptic communication
As non-synaptic message transmitters, astrocytes form a network in the neuropil called the astrocytic syncytium. Diffusion of molecules occurs in the syncytium via gap junctions between astrocytes.
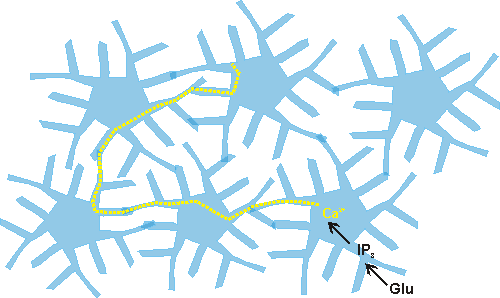
Research has found that calcium waves propagate through the syncytium (Cornell-Bell et al., 1990), and that these waves can be induced by mechanical stimulation and by the neurotransmitter glutamate. The calcium waves could result in long-distance modulation of the composition and concentration of molecules in the extracellular space if the flux of calcium into astrocytes throughout the network leads to calcium-sensitive release and uptake of ions and neuromodulators. The astrocytic syncytium allows a non-synaptic means of communication within the brain. Astrocytes can sense and integrate information from a number of synapses simultaneously and can also receive information about the composition of the extracellular space and within the blood vessels. While astrocytes, unlike neurons, are incapable of being potentiated, they do have a capacity for long-range modulation and integration, and may therefore serve an integrative role in brain functioning (see Blomstrand et. al., 1997).
Providing metabolic factors for neurons
One means by which astrocytes are involved in synaptic function is by providing metabolites for neuronal activity. The main energy source of the brain, glucose, enters the central nervous system through astrocytes in contact with blood vessels. The astrocytic processes which contact blood vessels are known as astrocytic end feet.
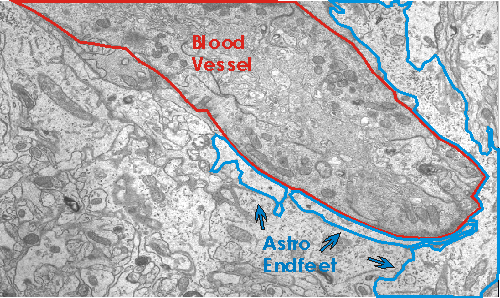
Evidence suggests that the glucose is partially metabolized by astrocytes and intermediates are released for neuronal use (reviewed in Pfrieger and Barres, 1996; Tsacopoulos and Magistretti, 1996). In the hippocampus, when glial metabolism is blocked by fluorocitrate, a glia-specific inhibitor of the Krebs cycle, synaptic transmission is inhibited (Keyser and Pellmar, 1994; reviewed in Pfrieger and Barres, 1996). This implies that the neurons are dependent on glia for fuel needed for synaptic function. In addition, evidence suggests that the amount of fuel supplied to neurons by the glia depends on the amount of synaptic activity. According to one finding, glutamate stimulates astrocytic uptake of glucose and subsequent release of lactate into the extracellular space (Pellerin and Magistretti, 1994; Pfrieger and Barres, 1996). Thus the neuronal activity both depends on and results in glial release of energy substrates.
Modulating ECS volume fraction through astrocytic swelling and filopodia extension
Astrocytes are an important determinant of the volume fraction of the extracellular space. Astrocytic swelling has been shown to occur as a result of both glutamate and adenosine receptor stimulation (Hansson, 1994; Bourke et al., 1983). Filopodial extension, or outgrowth of membrane, can be induced in astrocytes by focal application of glutamate (Cornell-Bell et. al., 1992). This filopodial extension can change the astrocytic size and morphology, possibly also altering the volume fraction occupied by extracellular space.
Synaptic function is sensitive to the extracellular volume fraction. As the volume fraction decreases, the concentration of components of the extracellular space increases and this could, theoretically, increase a neuron’s responsiveness to a given amount of neurotransmitter inputted. In fact, it has been shown that when the extracellular volume fraction is decreased as a result of increasing the concentration of K+, which is known to induce astrocytic swelling, the excitability of the neurons increases to the extent that seizures result (reviewed in Porter and McCarthy, 1997; Traynelis and Dingledine, 1989). Also, furosemide, which is known to block astrocytic swelling, has been recently shown to prevent electrically induced seizures (Hochman et al., 1995; Porter and McCarthy, 1997). These results suggest that astrocytes, through modulation of their size, can influence the excitability of neurons.
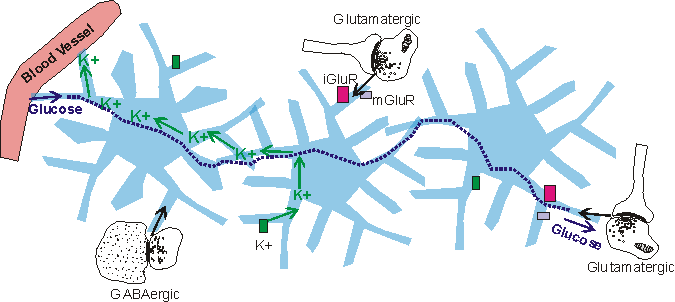
Ion channels, and neurotransmitter receptors
Astrocytes contain a wide variety of ion channels and neurotransmitter receptors and transporters. The K+ and Ca2+ ion channels are used by the astrocytes to maintain brain homeostasis for these ions. During synaptic activity, there is a buildup of K+ in the extracellular space, which is alleviated by astrocytic uptake (Nilsson and Hagberg, 1997). The astrocytes then eject the uptaken K+ into the capillary blood (Nilsson and Hagberg, 1997). The role of astrocytic Ca2+ ion channels is as of yet unclear.
In addition to ion channels, astrocytes contain neurotransmitter receptors. Before 1980 (Van Calker and Hamprecht, 1980), these receptors had only been observed in neurons, and were not thought to occur on astrocytes. The receptor types found in astrocytes are similar to those found in neurons. Among the astrocytic receptors are glutamatergic, GABAergic, adrenergic, serotonergic, and muscarinic receptors, each with differential expression across astrocytes and in different brain regions, and each coupled to a second messenger cascade (reviewed in Kimelberg, 1995; Porter and McCarthy, 1997).
Possibilities for influencing neuronal excitability
In addition to changing cell volume, astrocytes have other ways of modulating neuronal excitability. For example, an astrocytic depolarization near a presynaptic terminal could lead to an increase in the amount of neurotransmitter released (Ronnback and Hansson, 1997). Also, if the transmitter release is Ca2+-dependent, then astrocytic control of the concentration of Ca2+ in the extracellular space would lead to changes in excitability (Ronnback and Hansson, 1997). Other possibilities exist as well. Research has, in fact, shown that developing neurons in culture do not achieve fully functional synapses until glia are introduced (Pfrieger and Barres, 1997), though the mechanism of action for this is not known.
Astroglial glutamate uptake and the possibility of inter-synaptic glutamate spillover
Astrocytes contain high affinity glutamate transporters that are critical in maintaining the extracellular glutamate concentration at sub-excitotoxic levels and thereby preventing neuronal cell death (Rothstein et al., 1994; Rothstein et al., 1996). Insufficient glutamate uptake by the transporters is believed to play a role in amyotrophic lateral sclerosis, Alzheimer’s disease, schizophrenia, and AIDS, to name a few. Astrocytic uptake of glutamate may also serve to fine-tune the time course of glutamate in the synaptic cleft, perhaps by terminating the synaptic signal (Mennerick and Zorumski, 1994). Additionally, astrocytes may mediate inter-synaptic spillover of glutamate.
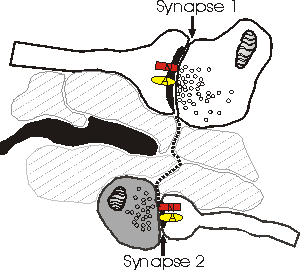
Depicted above is a hypothetical model explaining why NMDA receptors (N) sense a different number of quanta than AMPA (A) receptors. Dotted line shows the path of glutamate as it escapes from the cleft of synapse 1 and reaches synapse 2. In this model, spillover occurs because there are no astrocytic processes (black) along the path to uptake the relased glutamate. The bouton of synapse 2 (darkened) is releasing little or no glutamate, and therefore the concentration of glutamate that reaches synapse 2 is sufficient only to activate the (high-affinity) NMDA receptors. One problem with this model is that the high levels of extrasynaptic glutamate would lead to a general excitotoxicity.
Since astrocytes contain high enough densities of glutamate transporters to effectively prevent glutamate from diffusing past them, the presence of astrocytes near the synapses may be an important determinant of whether or not spillover occurs. In the hippocampal CA1 area, short inter-synaptic distances have indeed been observed, without any intervening structures to uptake glutamate (Ventura and Harris, 1999). Alternatively, since astrocytes grow towards released glutamate (Cornell-Bell et al., 1990), it is possible that only synapses with astrocytic processes at their axon-spine interface are releasing glutamate.
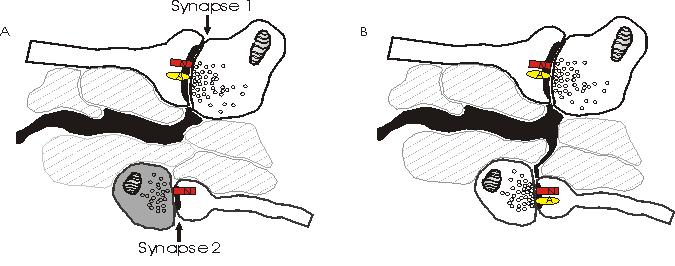
Depicted above is an alternative model explaining why NMDA receptors (N) sense a different number of quanta than AMPA receptors (A). Synapse 2 in (a) has little or no pre-synaptic release of glutamate and only NMDA receptors post-synaptically. When synapse 2 spontaneously releases quanta of glutamate, the NMDA receptors detect this, while there are no AMPA receptors present to sense the glutamate. The amount of release at synapse 2 in (a) is insufficient to spill over to neighboring synapses, and the astrocytes (black) prevent spill over from the active synapse 1. In (b), astrocytic processes have grown along a concentration gradient of glutamate towards the newly releasing synapse 2. Simultaneously, synapse 2 has recruited AMPA receptors post-synaptically.
This model, the astrocytic growth/AMPA receptor recruitment model, argues against spillover. Instead, it is possible that the discrepancy of the number of quanta sensed by NMDA and AMPA receptors may be explained by recruitment of AMPA receptors to the post-synaptic densities as the synapse becomes active (reviewed in Malenka and Nicoll, 1995).
References
- Blomstrand F., Khatibi S., Muyderman H., Olsson T, Ronnback L (1997) Calcium wave communication within the astroglial network via gap junctions. In: On astrocytes and glutamate neurotransmission (Hansson, Olsson, Ronnback, eds.), pp 121-143. New York: Chapman and Hall.
- Bourke R.S., Kimelberg H.K., Daze M., and Church G. (1983) Swelling and ion uptake in cat cerebrocortical slices: control by neurotransmitters and ion transport mechanisms. Neurochem. Res. 8:5-24.
- Cornell-Bell A.H., Finkbeiner S.M., Cooper M.S. et al. (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247: 470-473.
- Cornell-Bell A.H., Thomas, P.G., and Caffrey, J.M. (1992) Ca2+ and filopodial responses to glutamate incultured astrocytes and neurons. Can. J. Physiol. Pharmacol. 70: s206-s218.
- Hansson E. (1994) Metabotropic glutamate receptor activation induces astroglial swelling. J. Biol. Chem. 269: 21955-21961.
- Hochman D.W., Baraban S.C., Owens J.W.M., and Schwartzkroin P.A. (1995) Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science 270: 99-102.
- Keyser D.O. and Pellmar T.C. (1994) Synaptic transmission in the hippocampus: critical role for glial cells. Glia 10: 237-243.
- Kimelberg H.K. (1995) Receptors on astrocytes--what possible functions? Neurochem Int. 26(1): 27-40.
- Kullman, D.M. and Asztely, F. (1998) Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. TINS 21(1): 8-14.
- Malenka, R.C. and Nicoll, R.A. (1997) Silent synapses speak up. Neuron 19: 473-376.
- Mennerick S. and Zorumski C.F. (1994) Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature 368(6466): 59-62.
- Nilsson M. and Hagberg G.B. (1997) Astroglial potassium and calcium channels: contribution to brain homeostasis and excitability control. In: On astrocytes and glutamate neurotransmission (Hansson, Olsson, Ronnback, eds), pp 69-92. New York: Chapman and Hall.
- Pellerin L. and Magistretti P.J. (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 91(22): 10625-9.
- Pfrieger F.W. and Barres B.A. (1996) New views on synapse-glia interactions. Current Op. in Neurob. 6:615-621.
- Pfrieger F.W. and Barres B.A. (1997) Synaptic efficacy enhanced by glial cells in vitro. Science 277(5332): 1684-7.
- Porter J.T. and McCarthy K.D. (1997) Astrocytic neurotransmitter receptors in situ and in vivo. Progr. in Neurob. 51: 439-455.
- Ronnback L. and Hansson E. (1997) Does the astroglial network perform qualitative modifications of neuronal messages? In: On astrocytes and glutamate neurotransmission (Hansson, Olsson, Ronnback, eds), pp 121-143. New York: Chapman and Hall.
- Rothstein J.D., Martin L., Levey A.I., Dykes-Hoberg M., Jin L., Wu D., Nash N., and Kunci R.W. (1994) Localization of neuronal and glial glutamate transporters. Neuron 13: 713-725.
- Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., and Welty D.F. (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16(3): 675-86.
- Traynelis S.F. and Dingledine R. (1989) Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J. Neurophysiol. 61: 927-938.
- Tsacopoulos M. and Magistretti P.J. (1996) Metabolic coupling between glia and neurons. J. Neurosci. 16: 877-885.
- Van Calker D. and Hamprecht B. (1980) Effects of neurohormones on glial cells. In: Advances in Cellular Neurobiology (Federoff F., Hertz L., eds), pp 31-67. Academic Press, NY.
- Ventura R. and Harris K.M. (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 19(16):6897-6906. (888K PDF)